Dissolved Oxygen (DO) is an important substrate in aerobic fermentations. Oxygen molecules must overcome a series of transport resistances before being utilised by the cells.
Pauline Doran (Bioprocess Engineering Principles – 2013) describes eight mass transfer steps involved in transport of oxygen from the interior of the bubbles to the site of intracellular reaction:
- Transfer from the interior of the bubble to the gas – liquid interface
- Movement across the gas – liquid interface
- Diffusion through the relatively stagnant liquid film surrounding the bubble
- Transport through the bulk liquid
- Diffusion through the relatively stagnant liquid film surrounding the cells
- Movement across the liquid – cell interface
- If the cells are in a floc, clump, or solid particle, diffusion though the solid to the individual cell
- Transport through the cytoplasm to the site of reaction
The relative magnitudes of the various mass transfer resistances depend on the composition and rheological properties of the liquid, the type and mixing intensity, the size of the bubbles, the size of cell clumps, interfacial adsorption characteristics and a number of other factors.
Summarizing the above:
- The major resistance to oxygen transfer is the liquid film surrounding the gas bubbles
- The most important property of air bubbles is their size
For optimum growth it is therefore important to maintain the dO2 above this critical level by efficiently sparging (gas injection, bubbling gas through) the fermenter with air or mixed additional with pure oxygen. Of course, to be effective, the mass transfer rate from the gas bubbles to the liquid broth must equal or exceed the rate at which growing cells, bacteria take up the oxygen. In general terms the air or mixed gas volume required is ranging 1-2 litre/working volume/minute.
The rate of transport is given by:
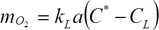
where kL is the oxygen transport coefficient (cm/h), a is the gas-liquid interfacial area (cm2/cm3), kLa the volumetric oxygen transfer coefficient (h-1), C* is saturated dO2 concentration (mg/l) (approx. 8 mg/l at 25 deg. C and 1 atm.), CL is the actual dO2 concentration in the liquid (mg/l), and NO2 is the rate of oxygen transfer (mg O2/ l/h).
kLa values depends strongly on the solution composition. Various solutes has a significant impact on the rate of oxygen transfer. During fermentation the coalescence properties vary and kLa can also be expected to vary accordingly.
kLa measurement for oxygen of cell culture bioreactors
Keep the temperature at 37 °C and agitation of the bioreactor at the maximum agitation rate recommended by the user manual. Sparge gas composed of 100 % nitrogen into the bioreactor at the maximum gassing rate of the bioreactor control system until the DO values drop below 10 %. Thereafter, stop agitating or set agitation to the minimum setpoint of the used controller, and flush the head space with 100 % air at 0.05 vvm via overlay gassing until the gas in the headspace has been exchanged at least three times. Continue headspace gassing and start data acquisition with a control software. Immediately start agitation and submerged gassing with 100 % air at impeller tip speeds and gassing rates of interest depending on needs, controller selection and bioreactor choice. For example, when using a small CellVessel Single-Use Bioreactor with a the Cronus-PCS devices, the impeller tip speed and gassing rate can range from 0.1 to 1 m/s, and from 1 to 60 sL/h, respectively. The measurement can be stopped when the DO measuring reaches values over 90 %. Carry out the experiment at least three times to provide a mean kLa of the bioreactor ideally with a standard deviation below 10 %. Repeat the kLa measurements under a broad range of process operation conditions varying gassing rates and agitation to evaluate the behavior of kLa at different cell culturing conditions.
